Accutest was founded in 1998 and has been offering BA/BE services to pharmaceutical companies in India and worldwide.
The company has achieved the highest quality standards, evident in its record of over 100 successful regulatory audits.
Read more

Healthy subjects and patients
(PK studies) for generics and biosimilars, incl. FTF and 505 (b)(2)

Phase II to IV studies for small/large molecules, PMS, Nutraceuticals, therapeutic equivalence, clinical end point
Characterization, Clinical comparability (PK / PD, studies), Immunogenicity studies for Biosimilar products

100+ regulatory
audits

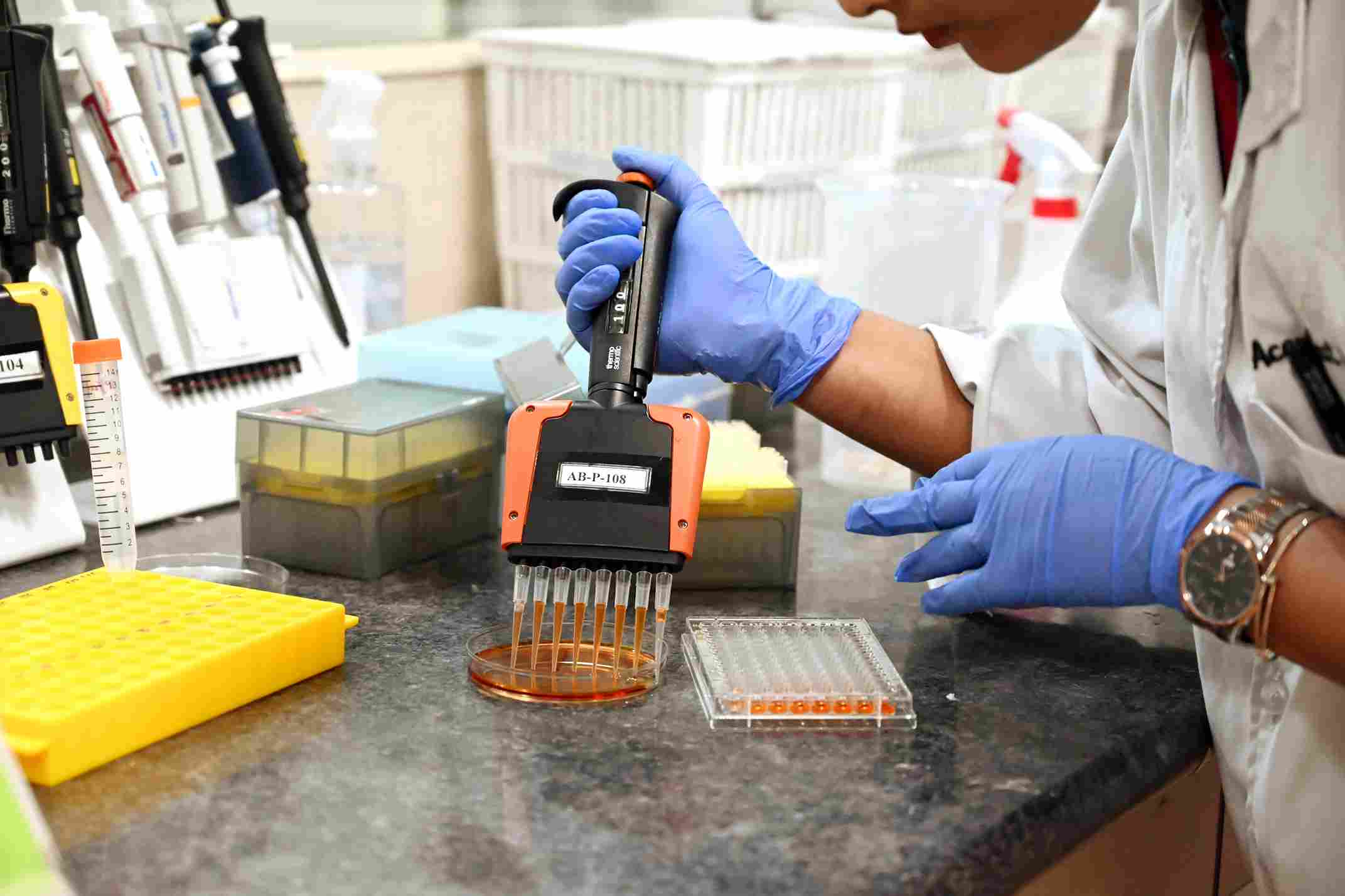
BA/BE lab in Mumbai with 26
LC-MSMS machines
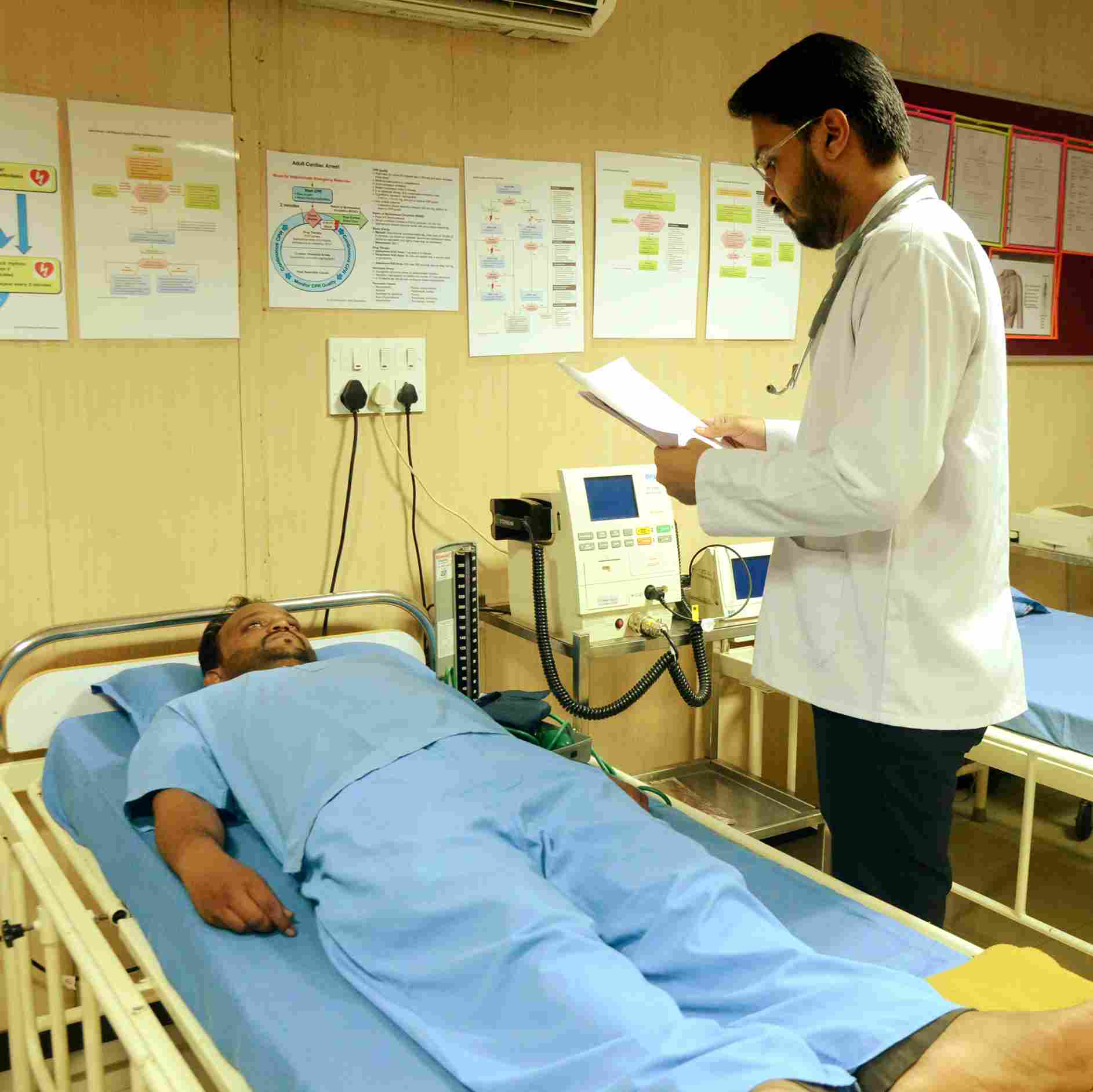
Three clinics with 354 beds in Mumbai, Ahmedabad & Vadodara

Over a 1000 validated
assays